Is Salt Ionic Or Covalent
In this article, "is NaF ionic or covalent" , the ionic or covalent properties of NaF with detailed explanations are discussed briefly.
Sodium fluoride is an inorganic species with greater ionic character. Information technology shows all the ionic character similar any ionic compounds. This salt is water soluble and white colored. It has different apply similar fluoridation of drinking water and pesticides, rat killer and also used to forestall cavities in teeth.
Some questions on sodium fluoride and its chemical behaviour are answered in this article.
Definition of Covalent and Ionic Compound
Covalent and ionic compound are the two most important species of Chemistry. All the molecules or chemical species are basically classified into these two compounds.
Sharing of electrons is the keyword of covalent compound because electronegativity departure between the two atoms is not large. Simply in ionic compound consummate sharing of electrons take place considering of the greater electronegativity difference betwixt two consisting atoms.

Image Credit: Wikipedia
To know more please check: 10 Ionic Bond Examples: Caption And Detailed Facts
How is NaF ionic?
NaF is consisted with 2 atoms one is sodium (Na), an electropositive metal and some other one is halogen chemical compound fluorine (F), an electronegative chemical element. Electronegativity of sodium is 0.93 (in Pauling scale) and for fluorine this value is three.98 (in Pauling scale). There is a huge electronegativity difference in sodium and fluorine (=3.05).
The large electronegativity deviation leads NaF to an ionic compound. The atomic number of sodium is 11 (1s2 2s2 2psix 3sone) and fluorine is 9 (1sii 2s2 2pfive). Sodium tin can hands donate its 3s electron to fluorine and exist unipositive sodium metallic ion and gain the nearest noble gas electron configuration. Similarly, fluorine tin accept the electrons donated by sodium to brand the electron configuration like the nearest element of group 0.
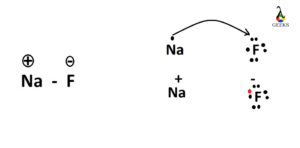
Thus, valence electrons of sodium is completely transferred to fluorine which is the main characteristics of any ionic chemical compound.
% of ionic graphic symbol (% of I.C) of any covalent and ionic chemical compound tin be determined using the following formula-
% I.C =16 ( χA – χB) + iii.five ( χA – χB)two
In this formula χA and χB are the electronegativity of atom A and B respectively.
Using this formula % of ionic character of sodium fluoride is –
- % I.C = 16 (3.98-0.93) + 3.5 (3.98-0.93)two
- % I.C = 81.35
This high value of % of ionic grapheme indicates that NaF is an ionic chemical compound.
To know more please follow: five Polar Covalent Bond Examples: Detailed Insights And Facts
Why NaF is non a covalent compound?
To exist a covalent compound, electronegativity difference between two molecules must be like or having bottom deviation. Thus, electron cloud is shared between two atoms. The electron deject is shifted more towards that cantlet having slightly greater electronegativity than the other atom.
In NaF, sodium is completely transferring its electrons to fluorine atom as fluorine is the about electronegative element and sodium is one of the most electropositive elements in periodic table.
From the in a higher place evidence it is clear that NaF is an ionic compound. Too of NaF has a greater amount of lattice energy.
To know more than please read: 4 Single Covalent Bond Examples : Detailed Insights And Facts
NaF lewis structure
Lewis structure is invented by Gilbert N. Lewis in 1916 in his article named as "The atom and the molecule". Lewis structure, also known as electron dot structure, is very much of import term to predict the bonded electrons as well equally nonbonding electrons.
To determine the lewis structure of whatever compound the following steps must be maintained-
- Valance electrons are shown effectually the respective atoms. Nonbonded electrons are not shown.
- Atoms must exist connected by single bonds. If the total number of electrons in any lewis structure and is 'n' and number of unmarried bonds are 'N', so (n-2N) number of electrons remain to be placed. These indicate the number of nonbonded electrons.
In this post-obit paradigm the lewis dot structure of water molecule is shown. Oxygen has total 8 electrons with 6 valance electrons. Between these six electrons, ii electrons form two sigma bonds with two hydrogen atoms and rest of the fours electrons are shown around the oxygen cantlet in this moving picture.
Sodium has its valance electron 1 and fluorine has its valence electron 5. Sodium donates its ane valance electron to fluorine and as a result fluorine has 6 valance electrons. So, fluorine has now total ten and valance electrons 6. These six electrons are shown around the fluorine atom.
To know more please go through: Is Peptide Bond Covalent : How Why, Comparative Analysis and Facts
Ofttimes Asked Questions (FAQ)
What happens when sodium fluoride reacts with water?
Answer: When sodium fluoride reacts with water, sodium hydroxide (NaOH) and hydrogen fluoride (HF) are formed. NaF + HiiO → NaOH + HF
Is sodium fluoride soluble in water?
Answer: Sodium fluoride is a polar inorganic compound and h2o is a polar solvent. Thus, sodium fluoride is soluble in water.
Is NaF a basic salt?
Answer: Yes, sodium fluoride is a basic table salt.
Is Salt Ionic Or Covalent,
Source: https://lambdageeks.com/is-naf-ionic-or-covalent/
Posted by: aldereteyetwall.blogspot.com


0 Response to "Is Salt Ionic Or Covalent"
Post a Comment